Policy Priorities
NCBIO advocates for the advancement of the life sciences industry in North Carolina so that it might meet its goal of serving the needs of its patients and customer. We promote the growth and development of the life sciences industry in the state and to ensure that its potential is maximized.
Updated February 2024
Areas of advocacy
To carry out our mission, we work closely with local, state and federal agencies to ensure the best possible outcomes for the industry, its stakeholders, citizens and patients. Our advocacy efforts include identifying issues that affect the life sciences industry, advocating for funding and policy change and working with stakeholders to ensure the industry is well-represented in the discussions that affect it.
NCBIO is committed to making sure North Carolina is a leader in the life sciences industry and that our stakeholders have a strong voice in the decision-making process. This page reflects the policy areas in which we are currently engaged.
State advocacy
Life Sciences Caucus
NCBIO collaborates with the North Carolina Life Sciences Caucus, a bipartisan legislative caucus in the North Carolina General Assembly dedicated to promoting the life sciences industry in North Carolina. The caucus works to identify and address the challenges facing the life sciences industry in the state and to create an environment that is conducive to the growth of the industry. The caucus also works to increase public awareness of the economic and social benefits that the life sciences industry brings to the state. The caucus is committed to creating an environment that will foster collaboration and innovation among the state's life sciences industry stakeholders, including universities, research institutions, industry partners and state and local governments.
The cochairs of the N.C. Life Sciences Caucus are Sen. Paul Newton, Sen. Mike Woodard, Rep. Donna White and Rep. Robert Reives.
North Carolina’s life sciences companies provide high-quality jobs with average annual wages in 2021 of $112,000, which is nearly double the $60,000 average for the private sector overall. A skilled and educated workforce is essential in the continued growth and success of the the industry in North Carolina.
From 2018 to 2023, life sciences employment in the state grew by 13%, compared to just 3% in the private sector overall. This growth, combined with low unemployment, has created an unprecedented demand for both workers and for the instructors who can train them. The state must focus on providing educational opportunities, job training and professional development for those interested in pursuing a career in the life sciences. Additionally, we should focus on creating pathways for individuals to enter the life sciences field, including career guidance and mentorship programs at all levels, as well as internships and apprenticeships.
By investing in career and technical education, we can ensure that the life sciences industry has a well trained workforce that is prepared to handle the challenges of the future. We must also work to create an environment that is welcoming and supportive of all cultures and backgrounds, so that everyone has the opportunity to contribute to the life sciences industry.
The One NC Small Business Fund provides matching funds to NC companies that receive federal SBIR/STTR awards. In the 2022 legislative session, the One NC fund was provided with recurring funds in the state budget for the first time. NCBIO supports increased funding for the fund and favors allowing companies to receive matching fund for multiple SBIR/STTR awards
When it comes to the ability of the North Carolina's infrastructure to meet the needs of the booming life sciences industry, the limiting factor is not power or transportation or available land, it is water.
Life sciences manufacturers use large volumes of water, usually for cleaning equipment. Wastewater from these manufacturing operations is usually discharged into municipal systems for safe treatment and disposal.
These municipal systems are typically designed to handle residential waste water. The added volume coming from an manufacturer can be more than a municipal system is designed to handle. Even if a treatment system can handle the volume, the local streams and rivers that receive the discharge may be challenged to accommodate the additional volume as those waterways also bear the burden of agricultural runoff, which is largely unregulated.
Waste water management in North Carolina is handled mostly at the local level with a patchwork of county and municipal authorities bearing the responsibility for treating and discharging the wastewater of their communities. The waste water in these local systems comes mostly from residential sources and are not designed to accommodate the needs of manufacturers.
NCBIO encourages legislators to provide the Department of Environmental Quality and Natural Resources with adequate staffing, especially the engineers needed to address the permitting backlog that currently exists. We also support and encourage the development of a statewide water-management strategy to increase wastewater discharge capacity.

North Carolina has 17 river basins that drain water from the state.
The North Carolina Biotechnology Center is a nonprofit, public-private partnership organization located in Research Triangle Park. Founded in 1984 by the North Carolina General Assembly, it was the first state-sponsored biotechnology initiative in the United States and merged the interests of the academic private and public sectors. NCBiotech's mission is to provide long-term economic and societal benefits to North Carolina through support of biotechnology research, business, education and strategic policy.
NCBiotech receives nearly all of its funding from the North Carolina General Assembly, and NCBIO encourages continued, robust support for this vital resource.
Access to investment capital is essential for the growth of the life sciences industry in North Carolina. Resources and investment capital allows companies to pursue innovative technologies and research, create new products and services, expand into new markets and hire more employees. Investment in the life sciences creates new jobs, improves public health and creates better treatments for a variety of diseases.
Qualified Business Venture Tax Credit
From 1989 to 2013, the North Carolina Qualified Business Venture Tax Credit provided significant tax benefits for investments in certain types of businesses. Individual investors were entitled to a tax credit equal to 25% of the purchase price paid by the investor for the equity securities or subordinated debt of the qualified business. The maximum credit was $50,000.
NCBIO is working with a growing network of angel investors to advocate for the reauthorization for the Qualified Business Venture Tax Credit or a similar mechanisms for encouraging investment and innovation across the state.
One NC Small Business Program
The One NC Small Business Program provides matching dollars to companies who win SBIR and STTR grants. For the first time, the program received $2 million in recurring funds for the 22-23 fiscal year after receiving only nonrecurring dollars in the past. Companies are currently limited to a single One NC Small Business grant per year no matter how many SBIR/STTR grants the company wins.
NCBIO advocates for increased funding of this program and for allowing companies to receive multiple matching grants if they earn multiple SBIR or STTR grants.
NCBIO is working for the repeal or reduction of the franchise tax, a tax applied for the privilege of doing business in the state. The tax is currently based solely on net worth. The state eliminated the calculations based on the value of real and tangible personal property or the taxpayer’s total actual investment in tangible personal property used in the past.
NCBIO favors doing away with the tax altogether or at least reducing its effect on prerevenue companies.
Federal advocacy
Acting as middlemen between drug makers, pharmacies, and insurers, pharmacy benefit managers exploit their market power to profit by driving up drug prices. Congress advanced PBM reform last year, with the House passing the Lower Cost, More Transparency Act by a bipartisan vote in December 2023.
The Pandemic and All Hazards Preparedness Act of 2006 awaits reauthorization. will help us get ready for the next pandemic. The original legislation was authored by Sen. Richard Burr (R-NC).
Each year, it is estimated that antimicrobial resistance claims nearly 25,000 lives nationwide and nearly 700,000 across the globe, according to the Review on Antimicrobial Resistance. The number of fatalities is expected to grow to 10 million annually by 2050. AMR, which reduces the effectiveness of treatments for infectious diseases, represents one of the most serious public health threats facing society. However, scientists have not developed a truly novel antibiotic in almost four decades.
The PASTEUR Act would address market problems to encourage the development of antimicrobials.
ARPA-H
The Advanced Research Projects Agency for Health is an NIH agency created by the Biden Administration to find innovative solutions to biomedical problems. ARPA-H received $1 billion in inaugural funding from Congress to improve the U.S. government’s ability to speed biomedical and health research to prevent, detect and treat diseases like Alzheimer’s, diabetes and cancer.
Congress ordered ARPA-H to set up offices in at least three places around the country, specifying that none of them could be the campus of ARPA-H’s parent agency, the National Institutes of Health, in Bethesda, Maryland. ARPA-H announced that it will open its first site in Washington, D.C. and plans to announce two additional locations later this year. NCBIO believes strongly that North Carolina should host the site for the region the state is in.
North Carolina has a demonstrated record of industries partnering with our institutions of higher education across the state to bolster our nation’s health workforce, leading breakthrough biomedical, biotechnology and life sciences research on a global scale. Our thriving economy, strategic location in the southeastern United States and low cost of living are attracting and retaining the world’s best and brightest. With an unmatched business environment, North Carolina offers a full complement of clinical, STEM, biotech and business training programs to prepare for a workforce skilled in cutting edge research, academia, health care and digital health for both public and private sectors.
Inflation Reduction Act of 2022
The Inflation Reduction Act (the continuing resolution passed by Congress at the end of 2021 to fund the federal government). One of its many provisions gives Medicare the power to dictate, not negotiate, the prices companies can charge for drugs and requires drug manufacturers to accept prices that other countries pay. The act also arbitrarily gives more favorable terms to biologics at the expense of small-molecule drugs.
We live in a world where a heart attack is now something that you can bounce back from. Where diabetes is something that patients live with every day. Where HIV is essentially a chronic condition. And that's the type of innovation that would not be possible without the biopharmaceutical industry.
President Biden and members of Congress made government drug price controls a key provision of their 2021 agenda. These well intentioned initiatives will have unintended consequences.
The U.S. market-driven approach has been the most successful framework globally for bringing medical breakthroughs to patients. The European market is a fraction of where it was because of the single payer in Europe. By putting price controls in, European governments have forced the European pharmaceutical industry to flee. And they're all setting up shop in the U.S., which is the only major market that's driving global innovation.
The law allows bureaucrats at the Centers for Medicare Services to arbitrarily set prices for certain medicines. If the manufacturer of the medicine doesn’t like the price offered, that’s fine. The government will instead hit the manufacturer with a 95% tax on the profits earned by the medicine.
Such an absurd policy will have a dramatic chilling effect on investment to create new treatments and cures. While American taxpayers may provide hundreds of millions of dollars for research through government agencies like the National Institutes of Health or the Department of Defense, the hard truth is that it takes hundreds of BILLIONS of dollars invested in life sciences companies to turn discoveries into treatments.
It comes down to investment. Pharmaceutical companies spend years and billions in resources with the understanding that if you hit a key milestone, whether it is simply proof of concept in humans or full FDA approval, there will be the resources available to get to the next milestone or that the company will be bought by a larger one to expand the capabilities and reward the team and investors. The entire biotech industry is based on these principles. And if the reward isn't there, the funding will dry up, and companies won't be able to bring new medicines to patients.
The Inflation Reduction Act disincentivizes the development small molecule drugs by granting them only nine years of protection from price "negotiations" while giving biologics thirteen years of immunity.
The Ensuring Pathways to Innovative Cures, or EPIC, Act would bring parity and move negotiation timelines for all new drugs out to 13 years. North Carolina Reps. Greg Murphy, M.D., and Don Davis (D-NC), along with Rep. Brett Guthrie (R-KY), introduced this bipartisan legislation in the House in February 2024.
While the IRA exempts drugs that treat orphan diseases from price "negotiations," that protection evaporates if the drug is approved for multiple indications (i.e., it can be used to treat something else). This perverse provision actively discourages drugmakers from studying their existing treatments to see if their use could be expanded to treat other conditions.
The ORPHAN Cures Act, making its way through Congress, would exempt orphan drugs from price controls even if they’re approved for multiple indications.
NCLifeSci supports the creation of an alternative, expedited pathway to coverage and payment for emerging devices and diagnostics. Transitional coverage for these technologies would bolster the innovation ecosystem and provide Medicare patients swift access to new technologies that existing therapies may be unable to address.
A recent Duke University study found it takes an average of five years for medical technologies to achieve nationwide coding, coverage, and payment. This delay hurts patients and increases costs.
The Centers for Medicare & Medicaid Services is working with industry stakeholders to create an alternative, expedited pathway to coverage and payment for emerging devices and diagnosticscalled Transitional Coverage for Emerging Technologies. NCLifeSci supports this effort.
Intellectual property and patents
The United States currently leads the world in the area of biotechnology because U.S. patent laws and legislation such as the Bayh-Dole Act of 1980 have provided favorable incentives to mitigate the high risks.
Prior to the Bayh-Dole legislation, federally funded research was owned by the government and offered for licensing on a nonexclusive basis or simply dedicated to the public. There was little incentive for businesses to undertake the financial risk to develop a product. The result was that only 5% of NIH-funded discoveries ever led to new or improved products.
Bayh-Dole also allows the federal government to “march in” under limited circumstances if a licensed invention is not being made available for public use, or during public health or other national emergencies. Some advocates say this provision also allows the government to intervene to set the prices of certain drugs that they believe are too high. NCBIO disagrees with this interpretation as Bayh-Dole was only intended to ensure that NIH-funding technologies came to market and provides no mechanism for reviewing prices.
The World Trade Organization is waiving certain intellectual property rights on COVID-19 diagnostics and therapeutics, following a decision to do the same for vaccines with the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights for COVID-19 vaccine technology. Any extension of this TRIPS waiver will compromise global vaccination efforts, undercut American innovation and jeopardize our ability to respond to future pandemics.
Every member of the Council of State Bioscience Associations, including NCBIO, signed a letter to President Joe Biden expressing serious concerns with the proposed expansion of IP protections for COVID technology.
Manufacturers are supplying therapeutics at a rate that outpaces demand. Biotech antiviral manufacturers have entered into dozens of voluntary licensing agreements with companies in South America, Africa and Asia to manufacture generic antivirals and distribute these products to countries throughout the developing world.
NCBIO opposes the TRIPS waiver for COVID vaccines and diagnostics. We encourage policymakers to consider and propose policies that more concretely address public health concerns to improve the management of COVID-19, such as strengthening health systems’ infrastructure, addressing vaccine hesitancy and supporting more robust COVID-19 testing and therapeutic procurement initiatives. Solutions to advance the global response to the COVID-19 vaccine cannot come at the expense of North Carolina’s innovation economy.
Product hopping
"Product hopping" is a name given to the practice of developing and releasing a new, improved (and often patent-protected) version of an existing brand-name medication. Some accuse drug companies of using new drug formulations as a tactic to blunt competition from generic rivals.
The Hatch-Waxman Act and related regulations define a process by which generic drug companies may seek expedited FDA approval to manufacture and sell counterparts to previously approved branded medications. Most product-hopping antitrust claims in effect assert that the branded manufacturer has gamed or manipulated the FDA’s regulatory scheme by opportunistically shifting resources to a new FDA-approved drug formulation, while, at the same time, withdrawing support for the prior formulation that faces imminent or nascent competition from generics.
Patent thickets
"Patent thickets" are a name given to overlapping patents that cover the same or very similar subject matter. Some advocates say that overcoming these thickets can be costly and delay less expensive competition from entering the market. An example often cited in the pharmaceutical is AbbVie's Humira, which has at least 136 patents that have been blamed for stalling biosimilar adalimumab competition in the United States.
However, Humira is very much an outlier. Excluding continuing U.S. market exclusivity for Humira and the etanercept originator, 21 biosimilars have launched referencing 8 other originator products. And for 20 of these biosimilars, the median time to launch post approval was 6.2 months, vs 13 to 14 months for generics approved between 2010 and 2014, according to BIO research.
It may be true that large “patent thickets” protect the innovations that went into developing and producing some of these originator biologics, but only select patents play a significant role in determining whether a competitor can bring a biosimilar to market.
According to BIO, 59% of patents “asserted” by originator companies in litigation are manufacturing process patents, 23% have to do with product indications, and 9% with formulation.
Environmental issues
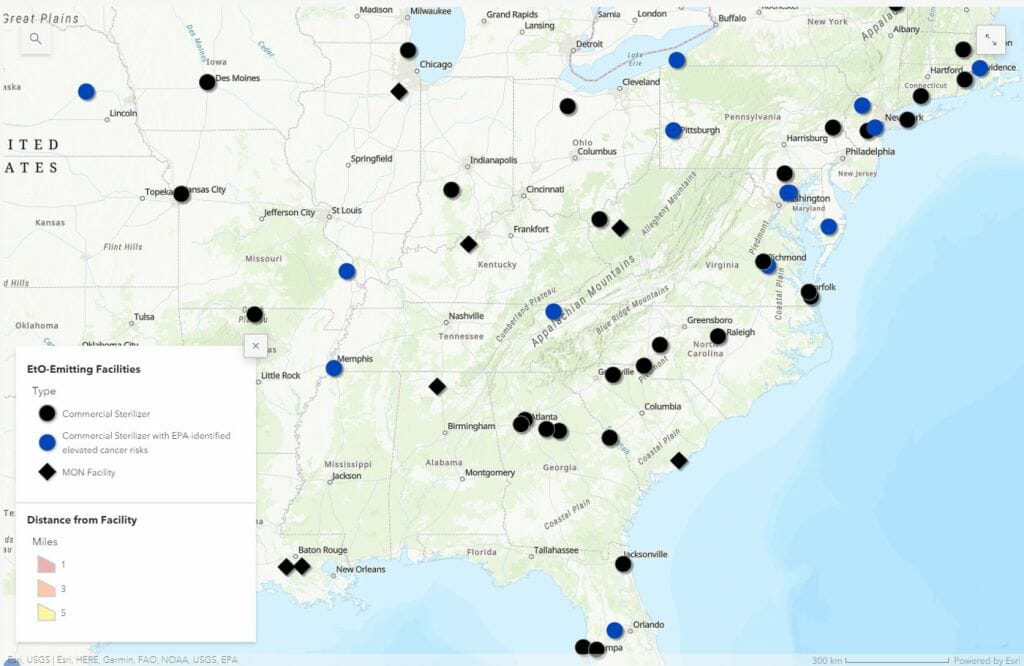
The EPA found that the chemical ethylene oxide, which used to sterilize roughly half of the more than 20 billion medical devices sold annually in the U.S. that require it, is contributing to elevated cancer risks in 23 communities close to sterilizing facilities.
NCBIO shares the EPA’s concern about the release of unsafe levels of ethylene oxide, but we are also cognizant of the fact that there are no adequate sterilizing alternatives to the gas for many medical devices. If even a small number of the approximately 100 sterilizing facilities decide to close rather than comply with new regulations, the supply chain for medical devices will be at risk.
NCBIO supports the FDA's efforts to work with device makers to reduce the amount of ethylene oxide used to sterilize their products and to encourage the development of new sterilization methods to ultimately replace ethylene oxide. The EPA must take the requirements of the medical device industry into account when proposing a rule limiting the emissions of ethylene oxide. The rule is expected in April 2023.
Commercial sterilizers in Eastern U.S. using ethylene oxide (2023)
Taxation
NCBIO urges immediate legislative action to repeal the harmful R&D amortization provision that went into effect in 2022. The 2017 Tax Cuts and Jobs Act changed the longstanding deduction for R&D expenditures to a mandatory five-year amortization for domestic R&D and fifteen-year amortization for foreign R&D, with the effective date of 2022.
As has been noted by companies and industries across the economy, the R&D amortization provision will have a negative impact on American innovation and high-paying R&D jobs. For the biotechnology industry specifically, it will divert much-needed funds away from small R&D-intensive companies, potentially doing long-term damage to the development of future treatments and ultimately limiting the pipeline of treatments and products that patients and consumers are relying on the life sciences industry to develop.
In January 2024, the House of Representatives passed a critical piece of legislation that would restore the R&D tax credit.
NCLifeSci worked with BIO to blanket Capitol Hill offices to make sure policymakers heard our industry’s voice on this matter. Letters were sent to the leadership of both the House of Representatives and the Senate, an online campaign flooded Congressional offices with messages of support and the media were leveraged to make sure lawmakers understood the importance of the full R&D tax deduction to the development of critical cures and innovations.
NCLifeSci convened a roundtable in August hosted by Alexandria Real Estate and attended by Rep. Wiley Nickel and staff representatives from the offices of Sen. Ted Budd; Rep. Gregory Murphy, M.D.; and Rep. Deborah Ross. The effects of the R&D tax penalty was one of the topics discussed.
The Senate introduced the American Innovation & Jobs Acts of 2023 (S. 866) in March 2023 that would restore the immediate expensing of R&D expenditures and remove this tax on innovation. The legislation was referred to the Finance Committee.
BIO Letter to Congress June 15, 2022