BMF presenters share lessons learned commissioning facilities during pandemic
At the NCBIO Biotech Manufacturers Forum held online Feb. 23, representatives from FUJIFILM Diosynth Biotechnologies, Lilly and Project Farma shared what they had learned about commissioning new facilities and equipment during the during the COVID-19 pandemic.
Presenting at the meeting were Scott Ewing, an engineering adviser for Lilly; Chris Cassada, a staff engineer with FUJIFILM and Christian Hermanas, Southeast lead for Project Farma.
Virtual FAT
In January 2020, Lilly announced it would invest up to $474 million in a new pharmaceutical manufacturing facility located in Durham that would create more than 460 new jobs over five years. The announcement coincided with the start of the COVID-19 pandemic in the U.S., and Lilly started sending people home. The last day in the office for many Lilly employees was March 6, 2020, Ewing said, but the company did not think the pandemic would last as long as it has.
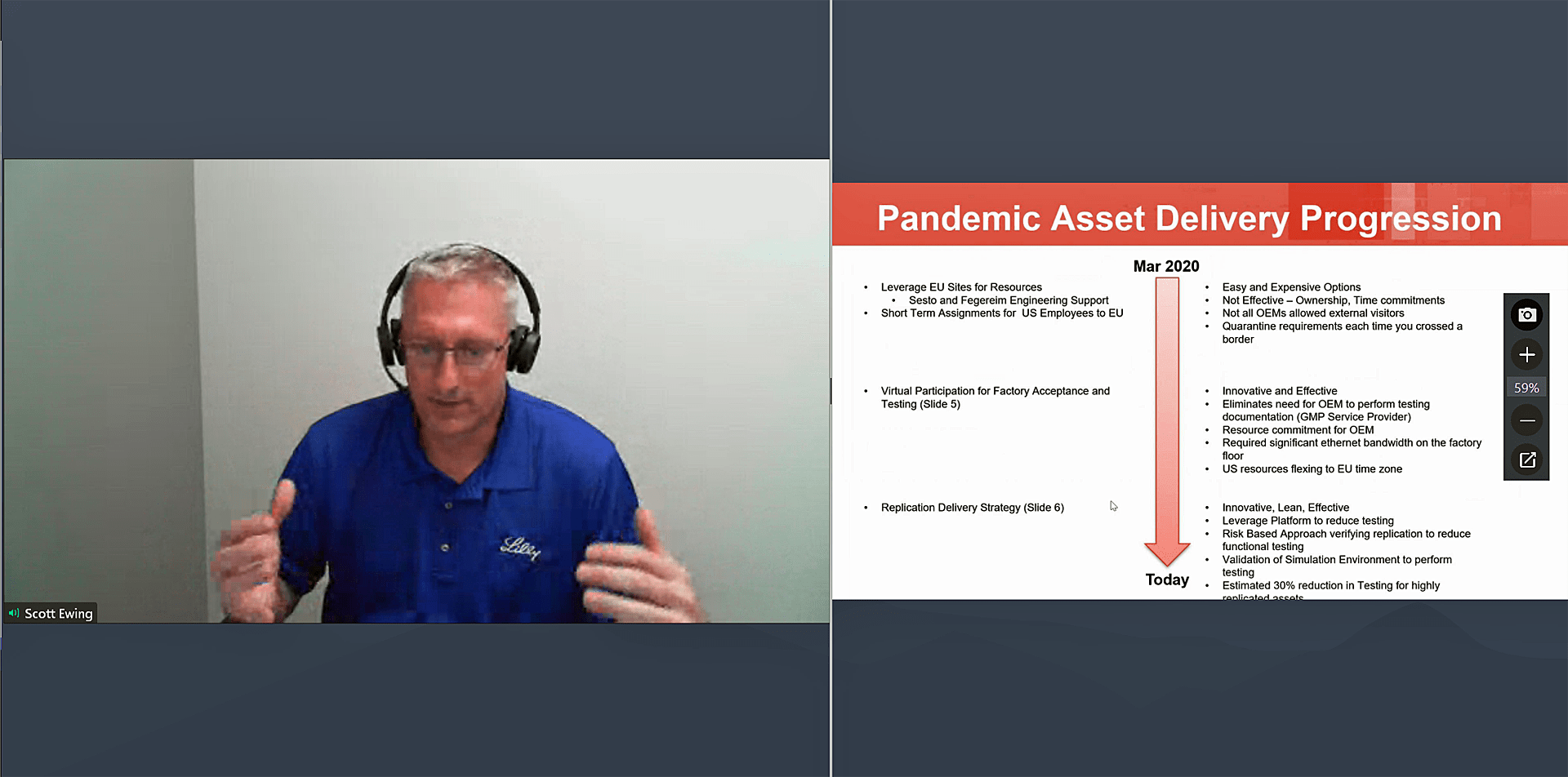
Initially, Lilly took the easy-but-expensive options of borrowing people and assets by leveraging its European sites for resources and assigning U.S. employees to sites in Europe to help keep processes moving. This strategy wasn’t effective, Ewing said, as many equipment manufacturers weren’t allowing visitors even if you could travel to them and traveling meant quarantine requirements every time an employee crossed a border.
Lilly worked to develop a system through which factory acceptance testing could be done virtually instead of having to be done on site. Video verification of testing became the norm with cell phones being used at the beginning and advancing to dedicated hardware using multiple cameras to document functional testing. The process was time consuming, but the company was stilling thinking short term, Ewing said.
In time, Lilly developed its Replication Delivery Strategy, a risk-based verification strategy and process used in conjunction with existing project and commissioning and qualification activities to reduce verification time of assets. The approach was innovative, lean and effective, Ewing said, and dramatically reduced the amount of testing needed for replications of an original system’s qualified design. Lilly estimates a 30% reduction in verification time can be achieved for highly replicated assets.
Communicate and document
On Feb. 25, 2020, FUJIFILM formally broke ground on a $54 million 32,000 square-foot expansion of its Bioprocess Innovation Facility in Research Triangle Park. A couple weeks later, schools and offices began closing, travel became more difficult and all nonessential FUJIFILM employees were required to stay home, Cassada said.
Initially, mask requirements were a challenge at the work site as the company tried to reconcile company, state and federal requirements. Compliance was good at the start driven by fear of the virus but began to wane as seasonal temperatures increased and the masks became more uncomfortable, Cassada said. The sheer number of contractors made communicating with them all difficult.
As work progressed, the main difficulty came from large pieces of equipment that had to be installed, including a homogenizer, centrifuge and a custom recovery system, all of which had to be connected to a new DeltaV distributed control system. Lead times grew longer as supply chains stalled.
FUJIFILM worked to develop new protocols for factory acceptance testing, asking question like is the equipment complex, is it new technology, what’s the vendor’s reputation and what happens if it doesn’t work? Based on the answers to those questions, the company could decide on no FAT, onsite FAT or remote FAT, the latter being the preferred pandemic option, Cassada said. The company worked to make the most of the situation by conducting thorough team reviews of FAT documents, extensively recording FAT testing (everyone using their cellphone) and including the recordings in the engineering turnover package.
Factory testing was not sufficient for all equipment, and site acceptance testing was needed for automated systems, custom equipment and critical equipment. To smooth out the process, FUJIFILM prescreened vendors, ensured communication between the vendors and installers and communicated travel policies well in advance.
Despite your best efforts, things will still go wrong, Cassada said. Prepare by ordering spare parts before you need them, and inspect everything. Review your bill of materials to identify items with long lead times and look for alternative suppliers. Pay close attention to your specifications because a good engineering design document will eliminate many issues, he said.
Collaborate and listen
Having positive and collaborative relationships with vendors is key to successful commissioning and qualification, Hermanas said. His employer, Project Farma, optimizes manufacturing and speed to market for therapies and has partnered with more than 80 life sciences organizations.
Hermanas shared a case study of managing factory acceptance testing for new equipment at a biologics facility. Due to pandemic travel restrictions, a traditional FAT with a full team onsite was not possible. The job included two major FATS, one with a new vendor and one with a trusted, long-time vendor.
The lack of a collaborative relationship with the new vendor led to miscommunication regarding design and completion and testing requirements, which cascaded into delays, increased costs and a damaged relationship with the vendor. The relationship with the trusted vendor allowed for many C&Q activities to be completed virtually, which decreased costs and kept the project on schedule, Hermanas said.
To maximize your chances of success working with a vendor, assign a process engineer to the FAT lead role to create a detailed schedule and coordinate meetings, Hermanas said. Use virtual meeting features like breakout rooms to have smaller discussions that would be difficult in the full conversation. Confirm and align on all testing between vendor and testing team and have the vendor provide a dedicated camera operator who is directed by the remote testing team. Finally, develop a risk acceptance strategy for issues and items that cannot be confirmed on camera. For example, you may decide to not worry about seeing every wire tag because they are easy to add or correct if missing or wrong.
Hermanas summarized the process of creating an effective virtual FAT as follows:
- Create and communicate a clear, detailed schedule.
- Prioritize collaboration and alignment with vendors.
- Develop a strong plan for communication and execution.
- Establish clear FAT roles and responsibilities.
- Maximize the power of virtual technology.
Done well, this remote process is as good as doing it in person, Hermanas said. Done poorly, it’s not worth doing at all.